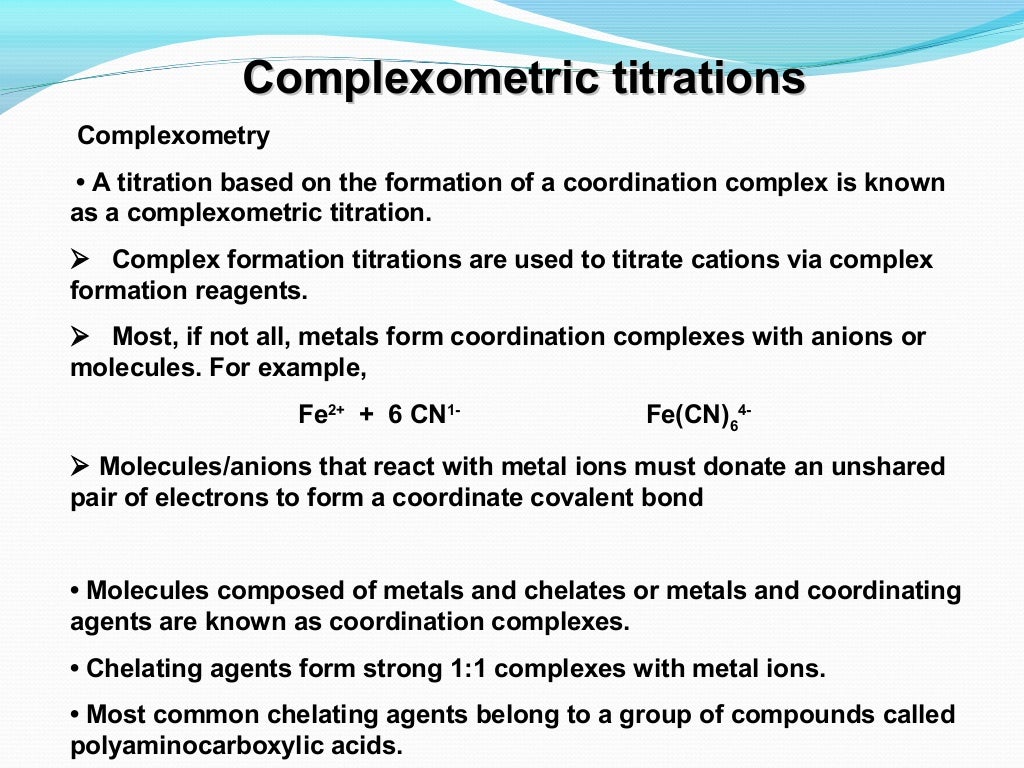
Complexometric Titration Buffer Solution Role . carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. Its adaptability allows for determining numerous. Distinguish among the various types of. For each of the three titrations, therefore, we can easily. This moves the position of equilibrium to the right. use the concept of chemical equilibria in complexometric titrations and calculations. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; The stoichiometry between edta and each metal ion is 1:1. complexometric titration holds immense importance across diverse fields within analytical chemistry. to do so we need to know the shape of a complexometric titration curve. thus, to prevent a ph change during the titration, the solution must be adequately buffered. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi.
from www.slideshare.net
Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. This moves the position of equilibrium to the right. Its adaptability allows for determining numerous. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. thus, to prevent a ph change during the titration, the solution must be adequately buffered. complexometric titration holds immense importance across diverse fields within analytical chemistry. Distinguish among the various types of. The stoichiometry between edta and each metal ion is 1:1. use the concept of chemical equilibria in complexometric titrations and calculations. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a.
Complexometric titrations
Complexometric Titration Buffer Solution Role complexometric titration holds immense importance across diverse fields within analytical chemistry. thus, to prevent a ph change during the titration, the solution must be adequately buffered. Its adaptability allows for determining numerous. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. For each of the three titrations, therefore, we can easily. to do so we need to know the shape of a complexometric titration curve. Distinguish among the various types of. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. complexometric titration holds immense importance across diverse fields within analytical chemistry. This moves the position of equilibrium to the right. The stoichiometry between edta and each metal ion is 1:1. use the concept of chemical equilibria in complexometric titrations and calculations. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed.
From www.studocu.com
Complaxometry COMPLEXOMETRY Complexometric Titrations CONTENTS Complexometric Titration Buffer Solution Role For each of the three titrations, therefore, we can easily. Its adaptability allows for determining numerous. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. Distinguish among the various types of. This moves the position. Complexometric Titration Buffer Solution Role.
From thechemistrynotes.com
Complexometric titration Complexometric Titration Buffer Solution Role use the concept of chemical equilibria in complexometric titrations and calculations. The stoichiometry between edta and each metal ion is 1:1. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. Its adaptability allows for determining numerous. Distinguish among the various types of. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a.. Complexometric Titration Buffer Solution Role.
From www.youtube.com
Buffers and Titration Curves YouTube Complexometric Titration Buffer Solution Role use the concept of chemical equilibria in complexometric titrations and calculations. thus, to prevent a ph change during the titration, the solution must be adequately buffered. For each of the three titrations, therefore, we can easily. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. This moves the position of equilibrium to the right. The stoichiometry between edta. Complexometric Titration Buffer Solution Role.
From mungfali.com
EDTA Titration Curve Complexometric Titration Buffer Solution Role complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. For each of the three titrations, therefore, we can easily. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. The stoichiometry between edta and each metal ion is 1:1. complexometric. Complexometric Titration Buffer Solution Role.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Complexometric Titration Buffer Solution Role thus, to prevent a ph change during the titration, the solution must be adequately buffered. For each of the three titrations, therefore, we can easily. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. use the concept of chemical equilibria in complexometric titrations and calculations. This moves the position of. Complexometric Titration Buffer Solution Role.
From www.coursehero.com
[Solved] Complexometric Titration PLEASE SHOW SOLUTIONS, THANK YOU Complexometric Titration Buffer Solution Role Its adaptability allows for determining numerous. Distinguish among the various types of. complexometric titration holds immense importance across diverse fields within analytical chemistry. thus, to prevent a ph change during the titration, the solution must be adequately buffered. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; to do so we need to know. Complexometric Titration Buffer Solution Role.
From facts.net
17 Intriguing Facts About Complexometric Titration Complexometric Titration Buffer Solution Role Distinguish among the various types of. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. use the concept of chemical equilibria in complexometric titrations and calculations. For each of the three titrations, therefore, we can easily. carrying out the reaction in a basic buffer solution removes the #h^+# as it. Complexometric Titration Buffer Solution Role.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Complexometric Titration Buffer Solution Role to do so we need to know the shape of a complexometric titration curve. use the concept of chemical equilibria in complexometric titrations and calculations. This moves the position of equilibrium to the right. Its adaptability allows for determining numerous. The stoichiometry between edta and each metal ion is 1:1. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and. Complexometric Titration Buffer Solution Role.
From www.youtube.com
Advanced Higher Complexometric Titrations YouTube Complexometric Titration Buffer Solution Role Distinguish among the various types of. complexometric titration holds immense importance across diverse fields within analytical chemistry. Its adaptability allows for determining numerous. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. carrying out the reaction in a basic buffer solution. Complexometric Titration Buffer Solution Role.
From users.highland.edu
Buffers Complexometric Titration Buffer Solution Role use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types of. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. This moves the position of equilibrium to the right. thus, to prevent a ph change during the titration, the solution must be adequately buffered.. Complexometric Titration Buffer Solution Role.
From www.numerade.com
SOLVED Why is the EDTA titration method called complexometric Complexometric Titration Buffer Solution Role to do so we need to know the shape of a complexometric titration curve. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. use the concept of chemical equilibria in complexometric titrations and calculations. Its adaptability allows for determining numerous. . Complexometric Titration Buffer Solution Role.
From psiberg.com
Complexometric Titrations Types, Advantages and Examples Complexometric Titration Buffer Solution Role Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. complexometric titration holds immense importance across diverse fields within analytical chemistry. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; to do so we need to know the shape of a complexometric titration curve. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in. Complexometric Titration Buffer Solution Role.
From www.numerade.com
SOLVED Please solve it 12. What is the role of EBT in the titration Complexometric Titration Buffer Solution Role Its adaptability allows for determining numerous. to do so we need to know the shape of a complexometric titration curve. use the concept of chemical equilibria in complexometric titrations and calculations. Distinguish among the various types of. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; For each of the three titrations, therefore, we can. Complexometric Titration Buffer Solution Role.
From gbu-taganskij.ru
SOLVED Complexometric Titration 10 ML Of MgSO4 Was, 59 OFF Complexometric Titration Buffer Solution Role carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. Distinguish among the various types of. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; Its adaptability allows for determining numerous. use the concept of chemical equilibria in complexometric titrations and calculations. to do so we need to. Complexometric Titration Buffer Solution Role.
From www.slideserve.com
PPT COMPLEXOMETRIC TITRATION PowerPoint Presentation, free download Complexometric Titration Buffer Solution Role use the concept of chemical equilibria in complexometric titrations and calculations. For each of the three titrations, therefore, we can easily. This moves the position of equilibrium to the right. Distinguish among the various types of. to do so we need to know the shape of a complexometric titration curve. complexometric titration holds immense importance across diverse. Complexometric Titration Buffer Solution Role.
From www.studypool.com
SOLUTION Complexometric edta titration curves Studypool Complexometric Titration Buffer Solution Role carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. The stoichiometry between edta and each metal ion is 1:1. use the concept of chemical equilibria in complexometric titrations and calculations. alkaline titration (e.g., ca2+, mg2+) • complex formation constant > 15; thus, to prevent a ph change during the. Complexometric Titration Buffer Solution Role.
From xi8.me
EDTA COMPLEXOMETRIA PDF Complexometric Titration Buffer Solution Role Slightly acidic titration (e.g., al3+, pb2+) 3+(fe3+ and bi. The stoichiometry between edta and each metal ion is 1:1. For each of the three titrations, therefore, we can easily. to do so we need to know the shape of a complexometric titration curve. thus, to prevent a ph change during the titration, the solution must be adequately buffered.. Complexometric Titration Buffer Solution Role.
From www.chegg.com
Solved Consider the following questions about buffer Complexometric Titration Buffer Solution Role The stoichiometry between edta and each metal ion is 1:1. Distinguish among the various types of. complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a. carrying out the reaction in a basic buffer solution removes the #h^+# as it is formed. complexometric titration holds immense importance across diverse fields within. Complexometric Titration Buffer Solution Role.